搜索结果: 'methocult media formulations for human hematopoietic cells serum containing'
-
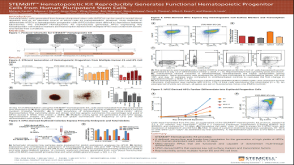 科学海报STEMdiff™ Hematopoietic Kit Reproducibly Generates Functional Hematopoietic Progenitor Cells from Human Pluripotent Stem Cells
科学海报STEMdiff™ Hematopoietic Kit Reproducibly Generates Functional Hematopoietic Progenitor Cells from Human Pluripotent Stem Cells产品类型:
Conference:
ISSCR 2017
产品号#:
04535
04545
05310
02692
09605
09655
产品名:
MethoCult™H4535富集无EPO
MethoCult™ H4535 Enriched,不含EPO
STEMdiff™ 造血试剂盒
StemSpan™红系扩增添加物 (100X)
StemSpan™ SFEM II
StemSpan™ SFEM II
-
 ArciTect™ 人 CRISPR 优化试剂盒 用于优化人细胞基因组编辑的完整试剂盒
ArciTect™ 人 CRISPR 优化试剂盒 用于优化人细胞基因组编辑的完整试剂盒 -
 EasySep™人细胞外囊泡(CD81)正选试剂盒
EasySep™人细胞外囊泡(CD81)正选试剂盒对来源于血浆、血清、细胞培养上清及尿液的人 CD81⁺ 细胞外囊泡(EVs)进行免疫磁珠正选分离
-
 EasySep™人Naïve CD4+ T细胞分选试剂盒 人Naïve CD4+ T细胞的免疫磁珠负选
EasySep™人Naïve CD4+ T细胞分选试剂盒 人Naïve CD4+ T细胞的免疫磁珠负选 -
 EasySep™人Naïve CD8+ T细胞分选试剂盒 人Naïve CD8+ T细胞的免疫磁珠负选
EasySep™人Naïve CD8+ T细胞分选试剂盒 人Naïve CD8+ T细胞的免疫磁珠负选 -
 EasySep™人静息CD4+ T细胞分选试剂盒
EasySep™人静息CD4+ T细胞分选试剂盒对来源于外周血单个核细胞或洗涤白细胞单采术样本的未标记的静息 CD4⁺ T 细胞进行免疫磁珠负选分离
-
 RosetteSep™人骨髓祖细胞预富集抗体混合物 免疫密度负选试剂混合物
RosetteSep™人骨髓祖细胞预富集抗体混合物 免疫密度负选试剂混合物 -
 RosetteSep™人脐带血祖细胞完全富集试剂盒 免疫密度负选试剂
RosetteSep™人脐带血祖细胞完全富集试剂盒 免疫密度负选试剂 -
 EasySep™人细胞外囊泡(CD9)正选试剂盒
EasySep™人细胞外囊泡(CD9)正选试剂盒对来源于血浆、血清、细胞培养上清及尿液的人 CD9⁺ 细胞外囊泡(EVs)进行免疫磁珠正选分离


 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒







 沪公网安备31010102008431号
沪公网安备31010102008431号