技术资料
-
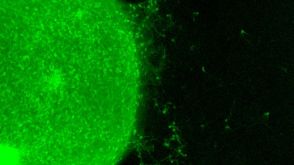 实验方案How to Generate AssemBloids™ from hPSC-Derived Dorsal and Ventral Forebrain Organoid Co-Cultures
实验方案How to Generate AssemBloids™ from hPSC-Derived Dorsal and Ventral Forebrain Organoid Co-Cultures研究方向:
疾病建模,神经科学,类器官
-
 科学海报Single-Cell RNA Sequencing Analysis of Regionally Patterned Human Pluripotent Stem Cell-Derived Neural Organoids
科学海报Single-Cell RNA Sequencing Analysis of Regionally Patterned Human Pluripotent Stem Cell-Derived Neural OrganoidsConference:
ISSCR 2021
-
 科学海报Generation of a Glia-Neuron Co-Culture System Derived From Human Pluripotent Stem Cells
科学海报Generation of a Glia-Neuron Co-Culture System Derived From Human Pluripotent Stem CellsConference:
GLIA 2021
-
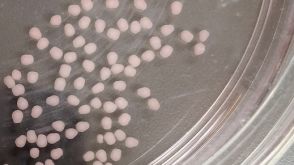 实验方案How to Dissociate 3D Neural Organoids into a Single-Cell Suspension
实验方案How to Dissociate 3D Neural Organoids into a Single-Cell Suspension研究方向:
干细胞生物学,疾病建模,神经科学,类器官,传染病
-
 实验方案How to Co-Culture Human Pluripotent Stem Cell (hPSC)-Derived Forebrain Neurons and Microglia
实验方案How to Co-Culture Human Pluripotent Stem Cell (hPSC)-Derived Forebrain Neurons and Microglia研究方向:
免疫,疾病建模,神经科学,传染病


 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒












 沪公网安备31010102008431号
沪公网安备31010102008431号