We have previously identified multipotent neuroepithelial (NEP) stem cells and lineage-restricted,self-renewing precursor cells termed NRPs (neuron-restricted precursors) and GRPs (glial-restricted precursors) present in the developing rat spinal cord (A. Kalyani,K. Hobson,and M. S. Rao,1997,Dev. Biol. 186,202-223; M. S. Rao and M. Mayer-Proschel,1997,Dev. Biol. 188,48-63; M. Mayer-Proschel,A. J. Kalyani,T. Mujtaba,and M. S. Rao,1997,Neuron 19,773-785). We now show that cells identical to rat NEPs,NRPs,and GRPs are present in mouse neural tubes and that immunoselection against cell surface markers E-NCAM and A2B5 can be used to isolate NRPs and GRPs,respectively. Restricted precursors similar to NRPs and GRPs can also be isolated from mouse embryonic stem cells (ES cells). ES cell-derived NRPs are E-NCAM immunoreactive,undergo self-renewal in defined medium,and differentiate into multiple neuronal phenotypes in mass culture. ES cells also generate A2B5-immunoreactive cells that are similar to E9 NEP-cell-derived GRPs and can differentiate into oligodendrocytes and astrocytes. Thus,lineage restricted precursors can be generated in vitro from cultured ES cells and these restricted precursors resemble those derived from mouse neural tubes. These results demonstrate the utility of using ES cells as a source of late embryonic precursor cells.
View Publication
 技术窍门组织解离方法指南
技术窍门组织解离方法指南 挂图SnapShot: Glioblastoma Multiforme Overview of the key concepts and mechanisms in glioblastoma multiforme biology
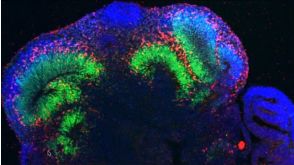
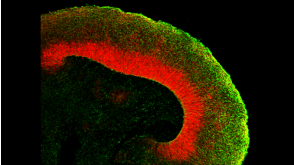
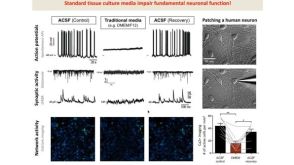
挂图SnapShot: Glioblastoma Multiforme Overview of the key concepts and mechanisms in glioblastoma multiforme biology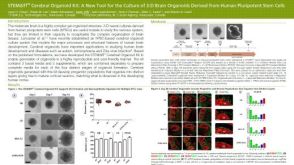 科学海报STEMdiff™ Cerebral Organoid Kit: A New Tool for the Culture of 3D Brain Organoids Derived from hPSCs
科学海报STEMdiff™ Cerebral Organoid Kit: A New Tool for the Culture of 3D Brain Organoids Derived from hPSCs

 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒







 沪公网安备31010102008431号
沪公网安备31010102008431号