技术资料
-
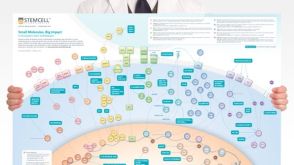 挂图Small Molecules, Big Impact in Pluripotent Stem Cell Research Overview of signaling pathways and small molecules in pluripotent stem cell research
挂图Small Molecules, Big Impact in Pluripotent Stem Cell Research Overview of signaling pathways and small molecules in pluripotent stem cell research -
 科学海报Efficient Cloning of Single CHO Cells Using a Novel Animal Component-Free Culture Medium Supplement
科学海报Efficient Cloning of Single CHO Cells Using a Novel Animal Component-Free Culture Medium SupplementConference:
ESACT 2013


 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒















 沪公网安备31010102008431号
沪公网安备31010102008431号